Figure 1
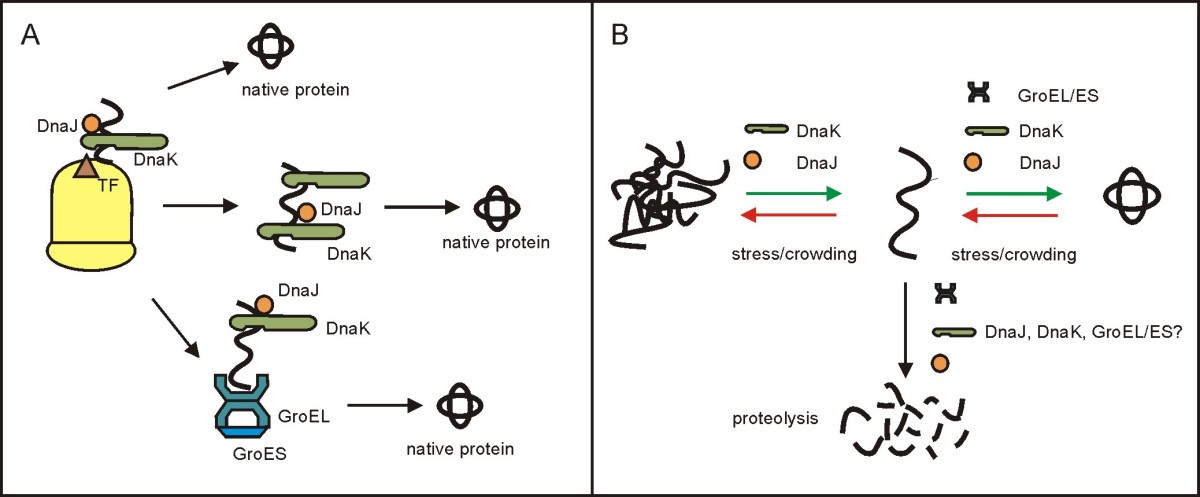
(A) Chaperone assisted folding of nascent and newly synthesized proteins in bacteria [34]. An elongating polypeptide is initially shielded by TF (trigger factor), a chaperone attached near the exit of the ribosomal tunnel. DnaJ (bacterial Hsp40) initiates binding of the polypeptide by DnaK (Hsp70) during translation. The initial, co-translational assistance of these chaperones is sufficient for the folding of many proteins (upper path). Other proteins are chaperoned by DnaK also after they detach from the ribosome (middle path). There are also proteins whose post-translational folding is assisted by DnaK and then by the GroEL/ES system. (B) Chaperone assisted reactivation of secondarily misfolded bacterial proteins. An unfolded protein can be rescued or directed to proteolysis. Participation of the molecular chaperones in recruiting single chains from aggregation and then in their correct folding is well documented. Possible cooperation of the chaperones with proteases has also been reported (see the text for more details). In bacteria, folding of both the primarily and secondarily unfolded proteins is assisted by the same chaperones.